Identify the Structure That Has Ortho-substituted Methyl Groups.
Isopropyl groups 6H doublet d. Those metal groups are um you know that methyl groups with benzene are electron donating groups.
Ortho Para Directing Group Chemistry Libretexts
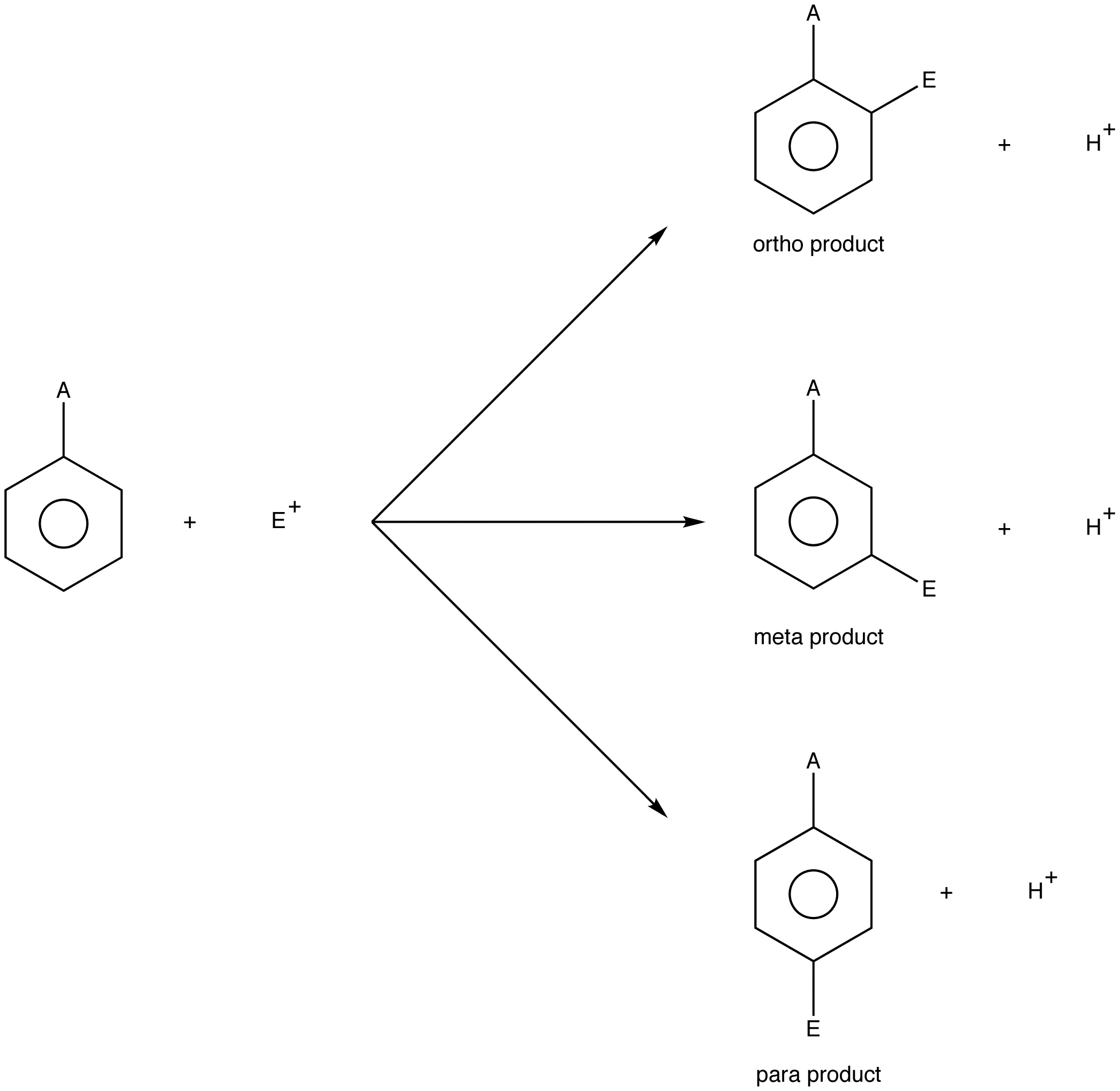
When directing effects of two groups reinforce the new substituent is located on the position directed by both groups.

. With both methyl groups in the axial positions. The structure of the herbicide sulcotrion has is shown below and is found to have a pK a of 313. Ortho meta and para historically carried different.
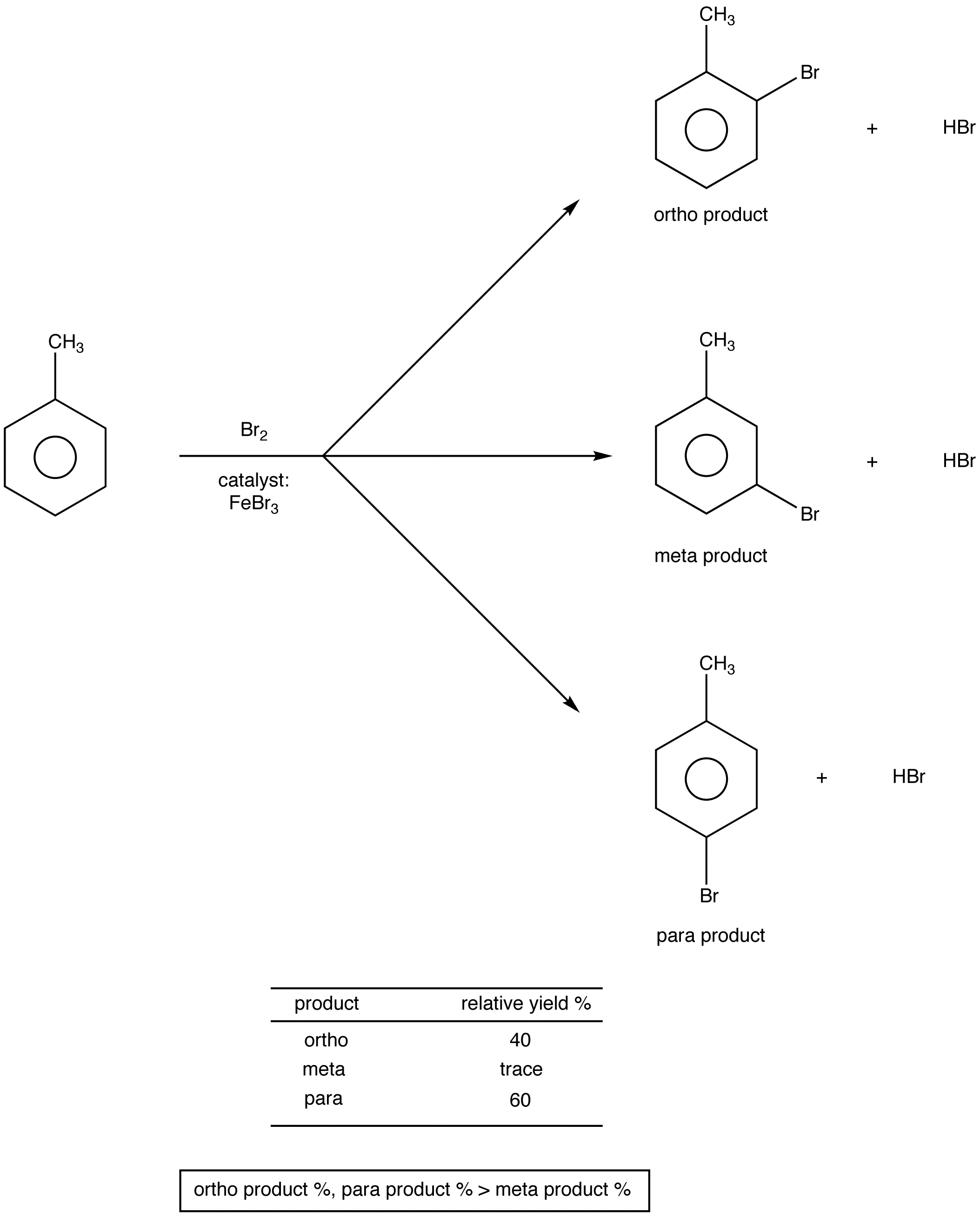
Electrophilic substitution in methylbenzene. So now the competition is between the ortho and para. With one methyl group in the axial and one in the equatorial position.
That carbon is bonded to an R group. Aryl groups chemical shift in the 7s a 4H or 5H integral depending on whether di- or mono-substituted b. Structure A is the more stable geometry though the difference in energy is small 0650pukcal mol1 at B3LYP6-311G Table 4.
Identify each as cis-or trans- 10. Chemistry questions and answers. H a and H b are ortho to one another adjacent.
2g resulting from the removal and migration of methyl groups respectively. J ortho 6-10 Hz. Integrates for only 1H and normally doesnt have the splitting that a CH hydrogen does 2.
For the ortho position of the benzene ring the J-value will be higher if a hydrogen atom is present. The prefixes derive from Greek words meaning correctstraight followingafter and similar respectively. H a H b and H c all couple to each other and have J values that correlate with the number of bonds between coupling protons.
I now have my nitrogen double bonded to this carbon. If the directing effects of two groups oppose each other the more powerful activator wins out. This is called ortho coupling and it couples with a J-value of about 6 to 9 Hz.
The nitration of methylbenzene. Clearly identify the bare and methyl-substituted naphtho-fused edges A and B in Fig. The carboxyl group trihalogenated alkyl groups and positively charged amino groups are also electron-withdrawing groups and act in a similar way.
Updated on October 02 2019. 1 are related by internal rotations of the 2 and 6-nitro groups and the methyl group. Ortho J meta H b td J ortho J meta or ddd J ortho J ortho J meta H c td J ortho J meta or ddd J ortho J ortho J meta H d dd J ortho J meta The appearance in the spectrum of two triplets of doublets indicates that J ab is almost equal to J bc and J cd.
-OH group is an ortho-and para-directing activator 50. The hydrogen atom α- to the three carbonyl groups is the most acidic due to the extensive resonance. So you know that amino groups NH two our strong activating species.
Applies to disubstituted Benzenes. No substitution occurs between two meta substitute nuts because of crowding. Identify the following as ortho- meta- or para- substituted.
J meta 1-3 Hz. Draw the structure of the conjugate base and explain the unusual acidity of this carbon acid. An exploration of the structureactivity relationship of these compounds based on the original hit N 4-butyl-5-iodo-6-methylpyrimidine-24-diamine 22a revealed that a butyl group at N 4 was ideal while substituting the iodo group with other halo groups such as fluoro chloro and bromo groups resulted in a decrease in potency.
Para-Identify the following as ortho- meta- or para- substituted. The nitrogen is bonded to a hydrogen and now my oxygen has three lone pairs of electrons around it giving it a negative 1 formal charge. Select the correct IUPAC name for the compound below.
We note that methyl from the closest carbon atom of the GNR backbone in agreement with the literature 41. Which structure has two methyl groups in the axial position. Substituent or side group names are based on the total of carbon atoms in the group and the position of attachment ie n-butyl sec-butyl tert-butyl.
Both in ortho and para there is a resonance structure where the positively charged carbon is next to the electron-withdrawing nitro group which makes the transition state more unstable. And the methyl group has a smaller effect on the stability of the sigma complex. The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative.
Ortho-Couplings Have a High J-Value. Ortho and Para have 4 resonance structures while meta has only 3 resonance structures. The unrelated racemic form has a C2c Z 4 structure which is a commensurate modulation of a P3c1 Z 2 parent structure typified by the room-temperature structure of Ru bpy 3 PF62.
With both methyl groups in the equatorial positions. CH 3 methyl groups based on clean 3H integration c. I have my benzene ring.
The nitrogen now is a plus 1 formal charge. From this information alone the signals can be identified as H a or H d and H b or H c as shown below. Identify the carboxylic acid that might be used.
The two stable molecular structures of TNT Fig. The carbon with the methyl group attached is thought of as the number 1 carbon and the ring is then numbered around from 1 to 6. So you have this Um this is a A You have a dimethyl amino group.
You number in a direction in this. 3 Identify substituent groups attached to the parent chain and place them in front of the parent name in alphabetical order. If you substitute a nitro group -NO 2 into the benzene ring in methylbenzene you could possibly get any of the following products.
This means we can delocalise charge easily in ortho and para which also means that these two are more stable comparing to meta positions. On the other hand CO molecules are. S O O O O Cl O sulcotrion Solution.
From the methyl H b and the hydrogen directly across the ring from the methyl group H c. H a and H c are meta to one another two carbons apart. So you can imagine that having to method groups instead of 200 wins is even mawr activating now.
We have shown through racemization kinetics studies that the enantiomerization barriers of the bis-ortho-methyl substituted Tröger bases 2 and 3 in acidic media are raised by 30 kJ mol-1. So if I go ahead and draw a resonance structure here. Chemistry questions and answers.
The closer the proton is to the other hydrogen atoms the greater the effect on the proton. Draw the structure of 13-dimethylcyclohexane in the chair form a. Substituent Effects in Electrophilic Substitutions.
Try to work from ends toward the middle. QUESTION ANSWER Identify the structure that has ortho abstituted methyl groups.
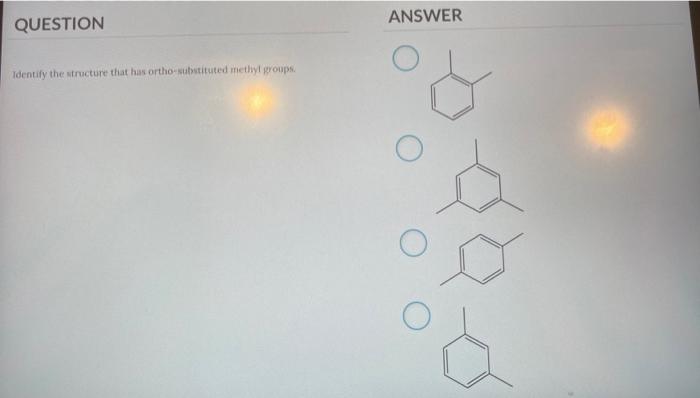
Solved Question Answer Identify The Structure That Has Ortho Chegg Com
No comments for "Identify the Structure That Has Ortho-substituted Methyl Groups."
Post a Comment